Pharmacovigilance
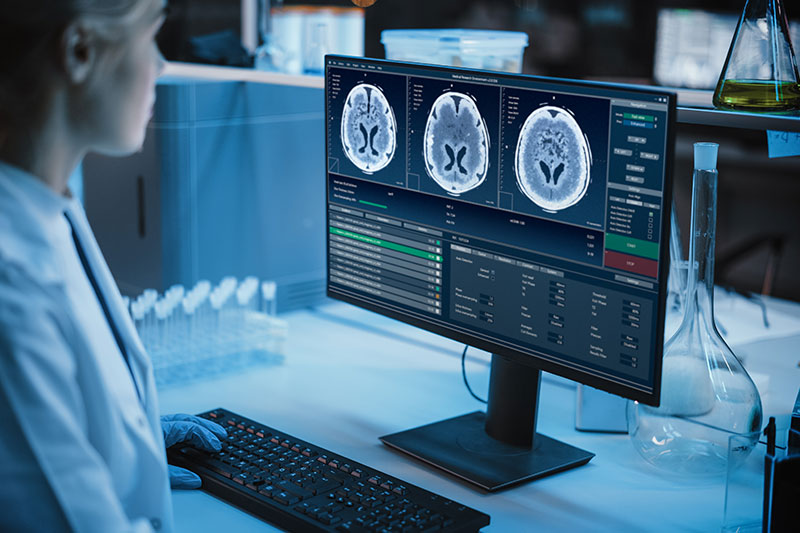
Patient safety is critical not only during product development but also for marketed products.
We understand the importance of patient safety, and have profound knowledge of the specific safety requirements associated with the use of radionuclides and imaging technologies.
Pharmacovigilance at ABX-CRO is led by a team of scientists and physicians who are able to fully support the stringent requirements of all aspects of Pharmacovigilance, from individual case management to writing regulatory reports and submissions.
Our pharmacovigilance evaluations are based on a validated and secure database system.
Our services include:
- Individual case management including follow up and submission to competent authorities
- Case narrative preparation
- Secure storage of cases in a validated database
- Medical writing for DSURs and PSURs
- Literature surveillance
- Investigator’s Brochure updates
- Risk/benefit assessment for your product